Before we do a deep dive into protein conjugation chemistry, let’s first describe what a conjugated protein is. In case that you are having issues understanding chemistry this igcse online chemistry tutor can help you out with your questions.
A conjugated protein is a protein to which another chemical group or molecule has been attached. Typically, covalent bonding interactions are used to conjugate molecules to proteins.
Proteins that contain only amino acids are generally referred to as free proteins.
Conjugated proteins can be attached to carbohydrates, lipids, organic complexes and may even have stabilized metal ions.
Conjugated proteins contain free proteins, as defined earlier, attached to molecules, called prosthetic groups. When carbohydrates are bound to a protein, the protein is called a mucoprotein or glycoproteins (considered to be the most abundant and largest conjugated protein). Some categories of conjugated proteins also take the form of proteolipids or lipoprotein. Proteolipids have the tendency to behave typically like lipids and are quite soluble in organic solvents. Lipoproteins (lipids attached to a protein molecule) behave typically like a protein. The lipid inside a lipoprotein imparts a lower density to the protein; which means a lipoprotein has a relatively low physical weight.
You can also have Metalloproteins and Chromoproteins, which are other derivatives of conjugated proteins. A metalloprotein has a metal group specifically bound to an amino acid moiety. A chromoprotein is a colored protein composed of a protein and a chromophore (functionality that gives them color).
A typical metalloprotein called hemoprotein is represented below. Note the way the iron ion is bound.
Hemoglobin is a conjugated metalloprotein that binds iron. Image from ScienceDirect.
Related articles:
- Biopolymer surface functionalization
- Immunofluorescence microscopy can be utilized to visualize proteins conjugated to fluorophores
Bifunctional Reagents for Protein Conjugation Chemistry
A bifunctional reagent features reactive or some very active end groups or molecules that make bioconjugation (crosslinking) possible. These bifunctional reagents can actively bind to compatible functional groups through their active end groups.
Research has shown four main functional groups make effective target moieties on proteins and prosthetic molecules. These functional groups include;
- Primary amines: A primary amine has an amino functional group (- NH2). We can have the aliphatic amine (R-NH2) and the Aromatic amine (Ar-NH2). Aromatic amines are more reactive and easier to conjugate to proteins than aliphatic amines.
- Carbonyls: Identified by the carbonyl functional group (R-C=O).
- Thiols (sulfhydryls): This is a functional group that has sulfur bonded to a hydrogen atom. They are usually denoted as R-SH.
- Carboxyls: Denoted R–COOH.
A closer look at all four bifunctional groups shows that they are also a part of amino acids, so they are easy to find on proteins.
Some common homo and hetero bifunctional reagents for protein conjugation. Image from SpringerLink.
Homobifunctional Reagents for Protein Chemistry vs. Heterobifunctional Crosslinkers
Bifunctional reagents can be homobifunctional or heterobifunctional.
Homobifunctional reagents have the same reactive group on either side and can be utilized for one-step protein conjugation chemistry. Heterobifunctional reagents have different reactive groups on each side and are typically utilized in multistep conjugation reactions with proteins.
Researchers have also utilized homobifunctional crosslinkers with N-hydroxylsuccinimide (NHS) esters to identify previously unknown interactions between proteins.
How to Use Bifunctional Reagents For Protein Conjugation
If you’re wondering how to utilize a bifunctional reagent for protein conjugation, in general you need to:
To use a bifunctional reagent for protein conjugation, first determine the target moiety on your protein and your prosthetic molecule. Then find a bifunctional reagent that will bind both your free protein and your prosthetic molecule. Finally, utilize the appropriate protein conjugation conditions to attach the protein to your linker and then conjugate your protein, with the crosslinker, to the prosthetic molecule.
Here are more details on the above general steps.
Step 1. Determine A Target Moiety On Your Protein First
Amino acids on proteins consist of carboxyl groups and amino groups. Since proteins are linear polymers consisting of amino acids, one end of the protein will have an unconjugated carboxyl and the other end will have an unconjugated amine group. The carboxyl end is called the C-terminus and the amine end is called the N-terminus. Both of these ends can be conjugated to. Protein labeling with fluorescent probes is a common reason to utilize the N- and C- termini.
You can quantify the N-terminus along with any free amine groups in a protein by reacting the protein with fluorodinitrobenzene (FDNB) or dansyl chloride. The fluorodinitrobenzene (FDNB) or dansyl chloride will link with any free amine inside the protein. If you notice that there are a lot of amines, then consider using bifunctional linkers with at least one NHS group to react to the amines on the protein. See below for lysine conjugation chemistry.
Other viable target moieties on your protein could include thiols from cysteine residues or the C-terminus carboxyl group. See the image below for amino acids that are easy to react with.
Lysine, Aspartic Acid, Cysteine, and Glutamic Acid are good target moieties for protein conjugation reactions due to their amine and carboxyl side chains. Image from NEB.
Related article:
- You may enjoy this article on methionine selective bioconjugation techniques.
Step 2. Determine a Target Chemical Moiety on Your Prosthetic Molecule
Conjugated proteins are proteins linked with chemical groups on other molecules, called prosthetic molecules. Prosthetic molecules can be carbohydrates, lipids or even ions.
You need to determine which chemical moiety on your prosthetic molecule you will conjugate to the protein of interest using the bifunctional reagent.
The best moieties on your prosthetic molecule to bind to are amines, carboxyl groups, or sulfhydryls as we stated previously.
If you can install an azide, tetrazine, or alkyne on your prosthetic molecule, these groups can make your protein conjugation chemistry really easy.
If none of these groups are available on your prosthetic molecule, consider converting a hydroxyl to an aldehyde to make it more reactive.
Typically nanoparticles that are commercially purchased can include functionalized polymer surfaces that you can conjugate to proteins. We discuss protein and antibody bioconjugation to gold in our article.
Azide-alkyne ‘click’ chemistry is a common method utilized for protein conjugation chemistry. It’s considered ‘bioorthogonal’ because azides and alkynes are uncommon in proteins. Image from Royal Society of Chemistry.
Learn more about orthogonal bioconjugation techniques in our related article.
Step 3. Find a bifunctional reagent that can react with both chemical moieties
Bifunctional reagents are important in the protein conjugation process as we’ve discussed previously.
The bifunctional reagent that you use to conjugate your protein to the prosthetic molecule must include chemical groups that can react with your protein (from step 1) and chemical groups that can react with your prosthetic molecule (from step 2).
If the two groups from Step 1 and Step 2 above are different, you need to use a heterobifunctional reagent. Western blotting can be utilized to prove that your conjugation reactions yielded higher molecular weight results.
Bifunctional reagents are also really useful for attaching proteins and other molecules to surfaces.
Using heterobifunctional reagents for protein conjugation chemistry
To use a heterobifunctional reagent, first react it with the protein. This reduces steric hindrance compared to the opposite case where you first react with the prosthetic group because your linker bifunctional reagent will be able to reach deeper parts of your protein without the prosthetic group interacting with the protein.
After reacting with the protein, you can react to the other functional group on the bifunctional reagent to react with your prosthetic molecule.
Note that two steps are involved for heterobifunctional reagents in order to avoid polymerization or side linkage of functional groups.
Using homobifunctional reagents for protein conjugation
Homobifunctional reagents can be utilized in one pot reactions containing both the protein and the prosthetic group or separately in two different conjugation reactions.
Site-specific protein conjugation
For site specific protein conjugation, researchers typically include unnatural amino acids inside their protein during expression. Cysteine groups could be used for site specific conjugation if only one disulfide bond is present in the protein and hence only those sulfhydryl groups are accessible to your bifunctional reagent.
Bioorthogonal reactions for protein conjugation
Azids, ketones, and aldehyde can be coupled to a protein via bioorthogonal chemistry. Typically, bifunctional reagents utilized to conjugate to these won’t react with amines or carboxyls on the protein. Ketones and aldehydes can be reacted with aminooxy or hydrazide compounds to yield a very stable oxime or hydrazone linkage between the protein and the biomolecule.
Lysine Conjugation Chemistry
Lysines are the most common targets for protein conjugation chemistry reactions. The amine on the lysine is essentially a nucleophile and reacts to amine-reactive chemicals such as N-hydroxysuccinimide (NHS) ester (these react via lysine acylation).
For successful Amine-NHS conjugation reactions, the pH of the reaction solution must be below 10.5, which is the pka of the ammonium group in lysine. This helps deprotonate the lysine groups. This means that the reaction should be carried out at ph around 8.5 to 9.0.
Lysine may also react with isothiocyanates and isocyanates and benzoyl fluorides.
Advantages of lysine-based conjugation
- Simple
- Commonly utilized for antibody conjugation
- Highly reactive, if they are accessible
- Versatile because it can react several cytotoxic agents (especially onto antibodies)
Bioconjugation strategies for lysine residues on proteins. Image from Nature.
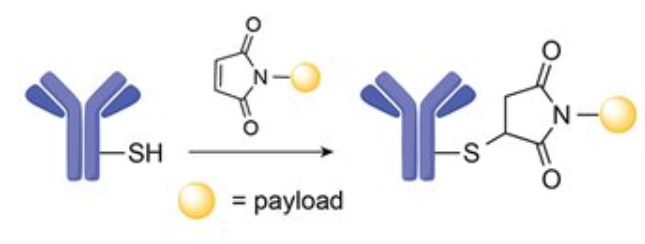
Maleimide Protein Conjugation Reaction Chemistry
You can react cysteines in proteins with maleimide groups as long as your buffer is in a pH between 6.0 and 7.0. A common approach is to reduce disulfide bonds in proteins first with DTT or TCEP and then to react with a maleimide-containing linker.
At a pH between 6.0 to 7.0, the maleimide group can react with sulfhydryl groups resulting in the production of a stable thioether linkage and the reaction is irreversible. At pH above 8.0 (alkaline), say 8.5, the reaction seems to favor primary amine and the rate of hydrolysis of the maleimide group to maleimide acid (not reactive) will increase.
You must eliminate compounds that contain thiol groups, like dithiothreitol (DTT) and β-mercaptoethanol (BME, aka 2-mercaptoethanol), from the reaction because they tend to compete with thiol groups on proteins. For instance, if you utilized dithiothreitol (DTT) to reduce disulfides in a protein and open up the sulfhydryl groups, you would need to thoroughly remove the DTT using a desalting column before you start the maleimide reaction. Instead of DTT, consider using the disulfide-reducing agent TCEP which lacks thiols.
You can quench free maleimide left over after reacting your protein by adding free thiols. You can also check your conjugation reactions using our HPLC Step By Step guide.
Advantages of Maleimide Protein Conjugation
- Thiols are only present in proteins on Cysteines
- For many proteins, one or two disulfide bonds exist, which mean that you can reduce them and conjugate in a site-selective manner using thiol-maleimide chemistry
- Cysteines can be readily introduced into proteins without affecting function, using site directed mutagenesis
Here are some strategies for using thiol-maleimide protein conjugation chemistry to create cleavable and permanent antibody drug conjugates. Image from RSC.
Protein conjugation protocol example
There are several protein conjugation kits that are available. Here’s an example from Vector labs. However, you can also utilize the chemical reactions above to create protein conjugates.
The steps below show a typical example of conjugating to lysines on a protein, based on information from GBiosciences.
Materials
- A PEG linker containing NHS such as any of these
- DMSO or DMF
- Amine free buffer at pH 7-8. Phosphate buffer works well
- 0.5-1.0 NaOH
- Protein containing Amines
- Desalting column
Procedure
- Dissolve 1-2mg the NHS ester reagent in 0.25 ml of DMSO or DMF
- Then add 2ml of phosphate buffer to the same reaction vial
- Add 100µl 0.5-1.0N NaOH to 1ml of the reaction from step 2. Vortex for 30 seconds
- Check that the pH is between 8.5 and 9
- Add your protein in pH 8.5-9 phosphate buffer to this vial
- Use a desalting column to remove any unreacted linker molecules. Here’s a step by step method for protein purification of recombinant proteins.
- Utilize the other end of the PEG linker to conjugate a prosthetic molecule