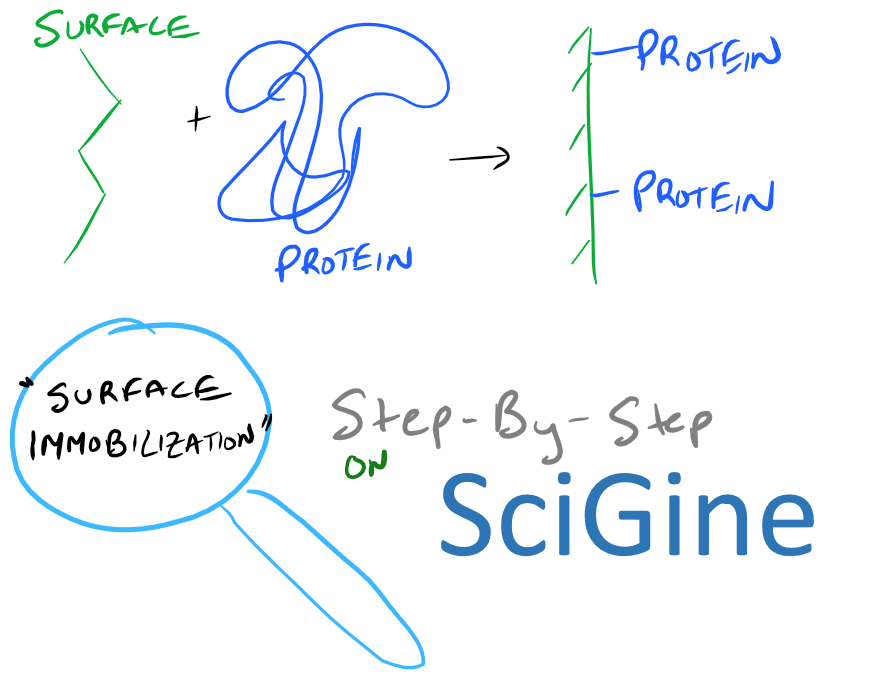
Introduction to Surface Functionalization of Biopolymers
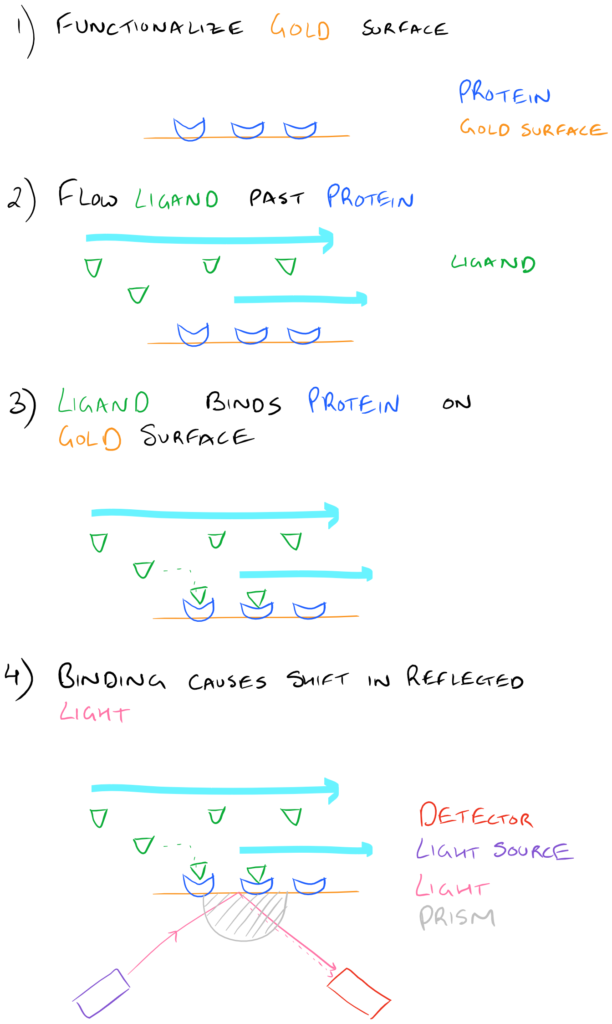
Immobilizing (or covalently attaching) proteins, lipids, carbohydrates, and other polymers on biopolymer surfaces is incredibly important for a number of reasons. Want your surface to be hydrophobic or hydrophilic? Want to attach interesting fluorescent molecules on a sensor surface? There are a ton of possibilities! One of the most common uses for biopolymer surface functionalization is Surface Plasmon Resonance. Here, a protein is covalently attached to a gold surface and several different ligands are flowed past the protein-surface. Researchers can then study the binding and unbinding of ligands to proteins on the gold surface and determine on/off rates etc.
For more information on methods for protein bioconjugation to gold, take a look at our article. We’ve also discussed techniques for bioconjugation to surfaces in a related article.
Take a look at the image below:
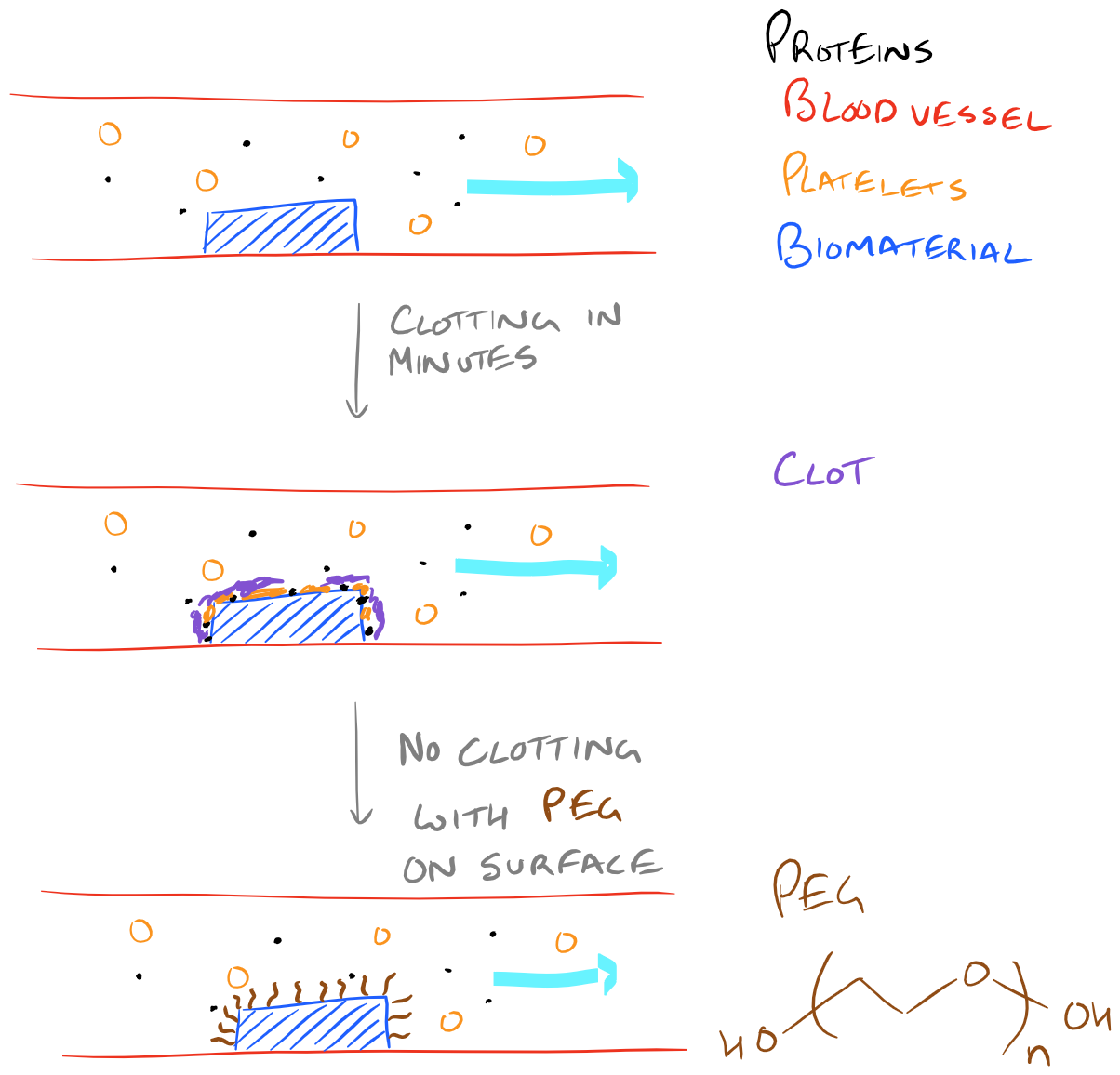
Another reason for immobilization of materials on a biopolymer surface might be to make it more hydrophilic. For a long time we have known that functionalizing long hydrophilic polymers on a surface can help prevent clotting and protein binding. This is one of the key methods for improving the blood compatibility of biomaterials. Without proteins to bind the surface and subsequent activation of platelets, biomaterials can be used inside the body for longer periods of time and they can even be implanted!
Curious about how you can tell if your protein attached to the surface? Label your protein with a fluorescent probe with our method!
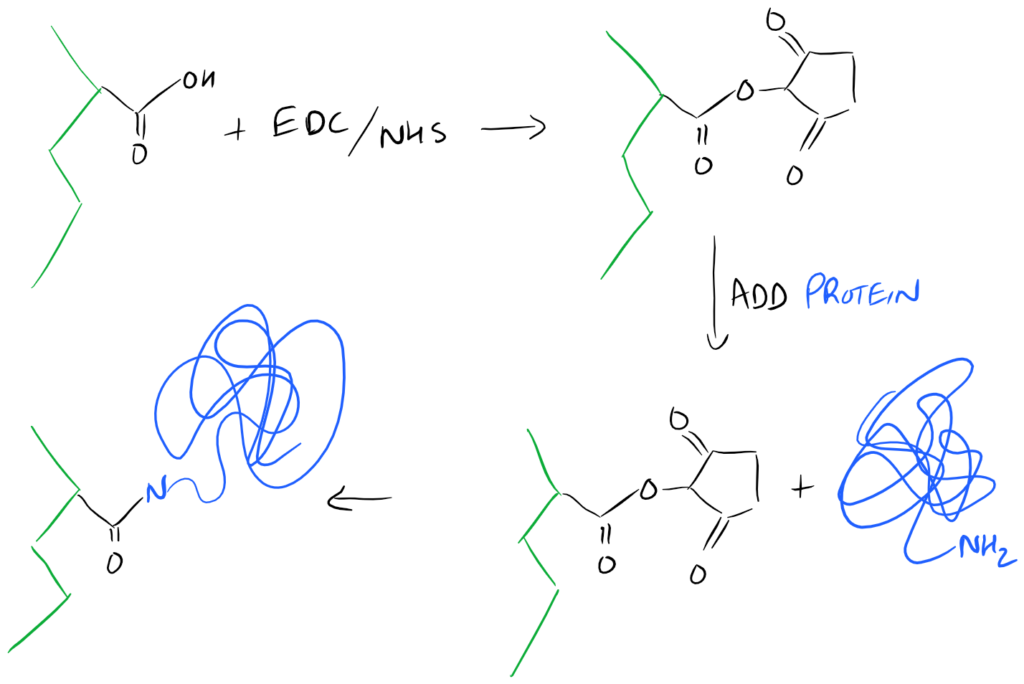
A Super-Simple EDC/NHS method for Surface Functionalization of Proteins on a Biopolymer
A common method for modifying the surface of a carboxyl-containing polymer with protein, is to attach the N-Terminus of the protein onto the surface. Here is a simple representation of the chemistry:
Materials for EDC/NHS Surface Immobilization of Proteins
- Coupling Buffer: We need to make sure that your protein is neutrally charged using an appropriate buffer (and your knowledge of the isoelectric point, pI, of the protein). Make a buffer with 100 mM Formic acid (pH 3-4.5) , acetic acid (pH 4.0 – 5.5), or maleic acid (pH 5..5 – 7.0) in water. Use NaOH for pH equilibriation.
- EDC [1-Ethyl-3-(3-dimethylamoniminopropyl) carboodiimide] at 0.4 M in water. Store at -20 C in small aliquots.
- NHS [N-Hydroxysuccinimide] dissolved in water at 0.1 M. Store at -20 C in small aliquots.
- Ethanolamine Hydrochloride dissolved in water at 1 M concentration, pH 8.5. Store at -20 C in small aliquots.
- Your Protein of Interest at 50 ug/ml in an appropriate buffer.
Step-by-Step Biopolymer Functionalization Methodology
- Wash the biopolymer surface with coupling buffer
- Thaw EDC, NHS, and Ethanolamine aliquots.
- Incubate the protein of interest with EDC and NHS at a 1:1 EDC:NHS ratio. Also, incubate the biopolymer surface with the EDC/NHS solutions. Leave at room temperature for 10 minutes.
- Wash the biopolymer solution with coupling solution 3 times.
- Add the protein + EDC/NHS solution onto the surface and incubate for 15 minutes.
- Wash the surface with coupling buffer
- Add ethanolamine solution onto the surface and incubate for 10 minutes
- Wash the surface with your protein storage buffer to re-equilibriate.
You can also utilize protein conjugation chemistry to impart unique tags onto your proteins that make them easier to functionalize onto surfaces.
Additionally, you can target specific residues on proteins like methionine residues for bioconjugation.
Notes on this Surface Functionalization Methodology
- If your biopolymer doesn’t have carboxyl groups, this methodology will not work. Choose an appropriate coupling technique based on the surface you’re trying to functionalize.
- EDC is hygroscopic and breaks down quickly in water. Keep the solid EDC under dry gas and if you have any EDC solutions, make sure to use them quickly or freeze them!
- Don’t reuse thawed EDC aliquots.
- You can change the incubation lengths to improve coupling efficiency between the protein and the surface.
Other Surface Functionalization methods on SciGine
- SPR with Surface Functionalized Carcinoembryonic antigen
- Biopolymer Functionalized with Fc/CgE on SPR
- BiaCore with SARS antibodies on a surface
- Oligonucleotide containing Microspheres Synthesized via EDC/NHS
- Biopolymer functionalization with a Cy5.5 fluorophore
Great Homebrew Video of Surface Modification
Literature References for Biopolymer Surface Functionalization
- Fischer MJ’s method for EDC NHS chemistry
- Review Article on Surface Functionalization
- MIT Lecture Slides on Surface Functionalization of Biopolymers