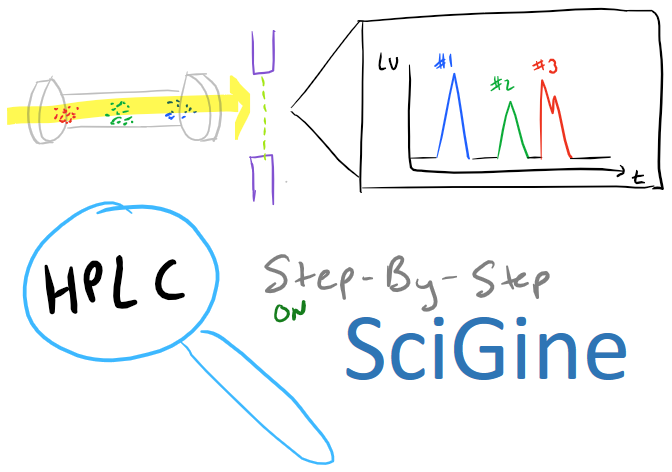
HPLC Method Overview
HPLC, or high performance liquid chromatography is an amazing analytical technique for chemical compounds including biopolymers, small molecules, and polymers. In this method, a sample is first dissolved to make a solution. This solution is then injected into a “column” that contains resin that will interact with the sample. This will slow down the movement of the sample through the “column” and as the sample comes out the other side of the column, it is detected. This allows you to know both the time at which the sample comes out and the intensity of the sample that was detected. Here’s an overview of this technique:
Note: If you’re writing research papers, I highly recommend Grammarly – it’s a free grammar check plugin for Chrome. Try it out here…
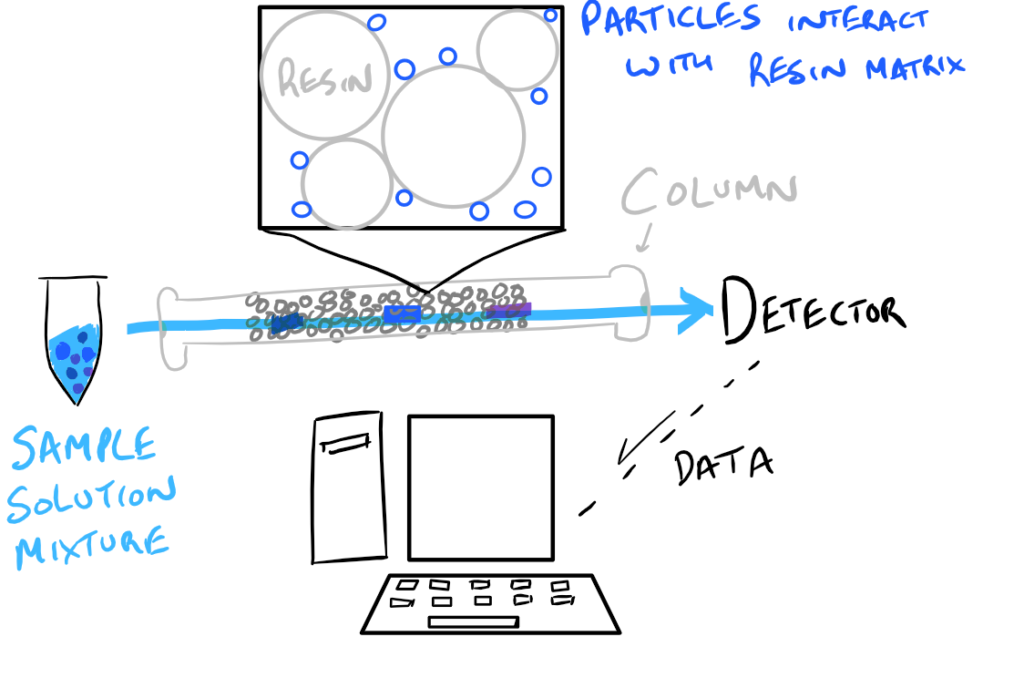
So, while there is continuous flow of some buffer through the column, we also inject our sample and observe as different molecules within the sample come out at different “retention times”. The detector on the end of the column can be any kind of detector but the most common types are refractive index (RI), ultraviolet (DAD), and fluorescence (FLD). Each of these will detect different properties of the molecules that come out of the column and display a chromatogram.
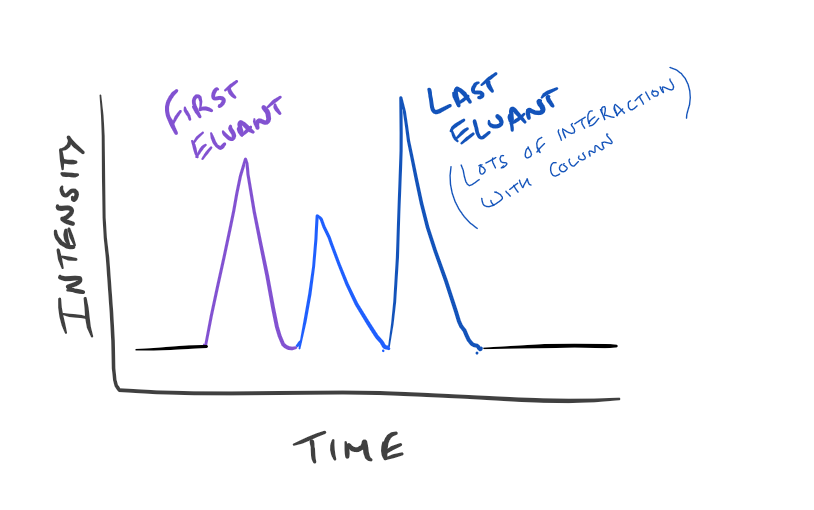
Types of Chromatography
Different column resin compositions determine the kind of chromatography that you are running and what molecules you can separate.
- Normal Phase: The column is filled with silica particles which are polar and the buffer running through the system is non-polar. Once you inject your sample, polar particles will stick to the silica more and have a longer retention time than non-polar molecules.
- Reverse Phase: The column is filled with hydrophobic particles (actually they are silica particles with long hydrocarbons on the surface). The buffer that is running through the system is polar (such as acetonitrile/water or methanol/water mixtures). This means that hydrophobic molecules will stick to the resin more and be retained longer.
Complete Step by Step HPLC guide
Materials
| HPLC autosampler vials | I only use autosamplers since manual injection is tedious 🙂 |
| Centrifugal filters with 0.2 um pores | To clean up samples |
| Eppendorf vials | For centrifuging |
| HPLC machine |
Methods
In a typical HPLC procedure you can decide the following variables:
| Variable | What it does |
| Flow rate | With fast flow peaks come out sooner but there’s they’re harder to resolve and tend to blend together. For more resolution, run slower. |
| Pressure | Affected by flow rate and solvent |
| Solvent Buffers | Determines signal intensity, how quickly the peaks come out, signal fidelity |
| Column Type | Determines the type of interaction with the sample |
| Detection Parameters | If using UV or FLD, you need to set the right excitation/emission wavelengths |
Since HPLC is a very machine-variable technique, I can only provide general guidelines.
For sample preparation:
- Dissolve your biopolymers or small molecules in a suitable solvent such as methanol
- Centrifuge at 10,000 rcf in an eppendorf vial and keep the supernatant to remove any large particular matter
- With a centrifugal filter, add 500 ul of your sample solution onto the top
- Centrifuge at 10,000 rcf and collect the filtrate (the solution that successfully passes through the filter)
- Load this sample into an HPLC vial
For setting up the HPLC machine:
- Make sure you have all your buffers set up
- Open the purge valve and purge the system for 5 minutes.
- Add your samples into the autosampler tray
- Stop the purge
- Close the purge valve
- Run the system at a normal flow rate (1 ml/min) with your buffer to equilibrate the column for 10 minutes
- Make sure that your pressure is stable (ie, less than 2-3 bar of fluctuation)
- Set up your sequence and your method
- Run a standard before your actual samples or as part of the same sequence
Example buffer system to determine Fluoresceinamine levels in samples:
Sample: Add 10 ug of fluoresceinamine into 1 ml of Acetonitrile.
Buffer: Pure acetonitrile buffer on a C-18 column; this is “reverse phase”.
Flow rate: 1 ml/min.
Column: 4.6 mm x 30 cm size.
Detection: Detect via a fluorescence detector set to Excitation @ 485 nm and Emission @ 535 nm.
Side-note: Use Grammarly to check your research papers – it’s a free grammar check plugin for Chrome. Try it out here…
Notes on HPLC methodology
- To clean the system and equilibriate it, you need to run enough solvent. However, this amount varies column-to- column. A typical 4.6 mm x 30 cm column should be clean when you follow the procedure above.
- Isocratic means that the solvent concentration stays constant throughout the run.
- It is useful to run standards before your samples as well as with your samples. Standards make it easy to identify which peak pertains to your molecule of interest.
- Always use HPLC grade solvents. This is especially true for solvents like THF which are frequently sold with inhibitors that also complicate your ability to detect your molecule of interest.
Applications of HPLC on SciGine
HPLC is such a versatile technique. Take a look at these methods on SciGine which assay different types of chemicals in various samples.
- Tocopherol (or tocopheryl quinone) measurement by HPLC with electrochemical detection
- Detection of N-t-butyloxycarbonyl-L-kyotorphin (Boc-KTP)
- Assay of LE, cellobiose-coupled LE (CcpLE) and its metabolites
- Amino acid incorporation into C600F bacteria
- Detection of urinary para-amino benzoic acid
References
ChemGuide Summary of Technique
Method Guide from Waters
Overview on Wiki
[…] end with a protocol for the MTT assay. It would be a great test of your skills if you could use our High Performance Liquid Chromatography (HPLC) Method Guide to detect the products of the MTT […]
i found it best ,makes my thoughts clear. thanks.
Thank you LR! Glad you like the blog post and read it.
[…] (SEC) works similarly to separate out larger molecules from smaller ones. Take a look at our HPLC Step by Step guide to understand chromatography in […]